Here at Springboard Biodiesel, we hear a lot of questions and erroneous information about what type of feedstocks can be successfully turned into biodiesel. The following “1000 words or less” should hopefully clear up a little bit of that. Note that the following post does not take into account impurities such as water and FFA content of various feedstocks.
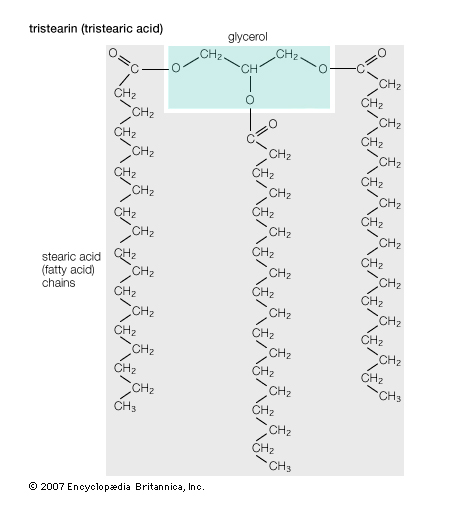
To begin with, the primary stuff that feedstock is made from is triglycerides. These triglycerides are composed of three long fatty acid chains attached to a glycerol molecule. Note that as shown in the figure below, the glycerol is only a very small part of the triglyceride molecule. Most of the bulk of the molecule (as well as the energy contained therein) is in the long hydrocarbon chains called fatty acid chains that are attached to it. As long as an oil or fat has this basic structure, it is a good candidate for being turned into biodiesel.
Are there any oils that don’t have this structure?
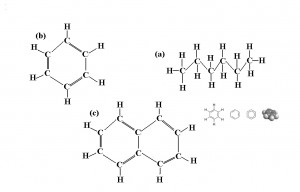
Sure! Take a look at some of the medley of things that you might find in petroleum diesel fuel.
Because the biodiesel reaction primarily happens at the points where the fatty acid chain meets the glycerol, the molecules shown above would obviously not be able to be turned into biodiesel.
Therefore, the primary factor of an oil or fat that determines if it can be turned into biodiesel is if it was initially composed of triglycerides. There are some natural oils such as orange oil that are not composed of triglycerides. Typically though, these are only found in trace quantities. Most natural oils that can be found in bulk are triglycerides. The following are some commonly asked questions that relate to this fact and further explain its ramifications.
1- What about saturated fats? Saturated fats are simply a specific type of triglyceride that has no double bonds along the carbon chain. The triglyceride example shown above is actually a saturated fat. Saturated fats can make excellent biodiesel. One drawback of biodiesel made from saturated fats is that it typically has a high melting point. Therefore, they are often solid at room temperature. We tested crude palm oil in our shop BioPro 190 at one time and had to literally use a shove to scoop the solid pieces of oil and put them into the machine. We then had to turn on the manual heaters to melt the oil before we could begin the process. It is also worthy of note that the solidified feedstocks will often be holding in suspension large amounts of water or contaminants that should be removed before processing. Also, the biodiesel made from saturated fats will tend to have a high melting point. Therefore, a biodiesel user may experience cold weather gelling of fuel. On the up side, biodiesel made from saturated fats will typically have a better oxidative stability and fewer NOx emissions than one made from unsaturated fats.
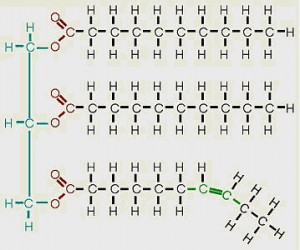
2- What are unsaturated fats? Unsaturated fats are fats or oils in which one or more of the carbon to carbon bonds in the fatty acid chain, is a double bond. Monounsaturated fats have only one double bond in the chain while polyunsaturated fats have more than one double bond. Below is an example of a monounsaturated fat.
The more highly unsaturated a fat is, the lower the gel point typically is. Therefore, unsaturated fats are excellent for cold weather biodiesel production and use. The downside of unsaturated fats is that they will often be more prone to oxidation and rancidification than their saturated counterparts. Typically, biodiesel made from highly unsaturated fats will require an oxidative stabilizer to be used safely as fuel.
3- What about trans-fats? Trans-fatty acids are fats and oils with a double bond in the carbon chain that is turned the other way than the typical cis-double bond. It is called a trans-double bond; hence the name trans-fatty acid. They are formed from intense heat of frying and from the process of partially hydrogenating unsaturated fats.
4- What are hydrogenated oils? Hydrogenated oils are oils in which atoms of hydrogen have been added to the vicinity of the double bond. This converts it to a single bond. A molecule of oil in which all of the double bonds have been hydrogenated is chemically identical to a standard saturated fat.
5- Can biodiesel be made from hydrogenated fats or trans-fats? Absolutely. A hydrogenated fat is chemically the same as a saturated fat. While trans-fats are thought to be bad for your health, they have no ill effect on the biodiesel reaction.
6- What are some examples of fats and oils that can be processed into biodiesel.
a) Beef tallow, coconut oil, palm oil, pork lard, and chicken tallow are all examples of feedstocks that are high in saturated fats that have been successfully utilized in biodiesel production. There is typically very few difficulties with oxidative stability for these feedstocks, however, their use often poses significant hurdles due to their high gel point.
b) Canola oil and olive oil typically monounsaturated and have very few issues with oxidative stability. They also tend to have a low gel point. These are often the most trouble free feedstock sources for biodiesel production.
Soy oil, corn oil, sunflower oil, various fish oils, rice bran oil, and hemp oil, are example of oils that are often quite highly unsaturated and will nearly always require the use of an antioxidant or stabilizer prior to use to avoid problems with polymerization and rancidification
Written By Daniel Bowen, Senior Chemist at Springboard Biodiesel